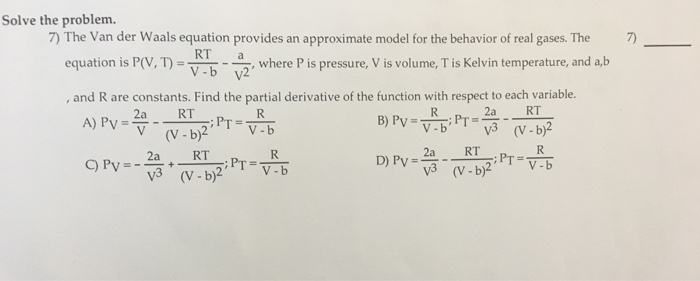
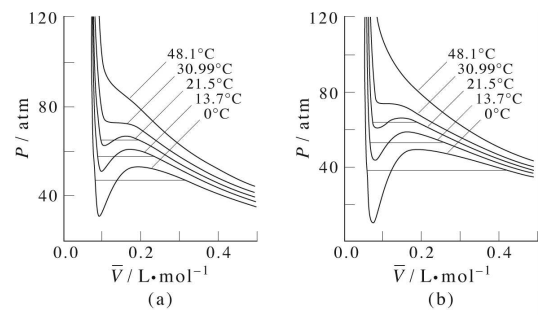
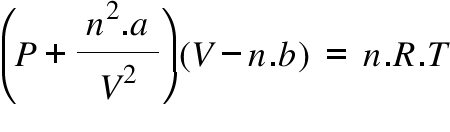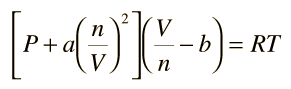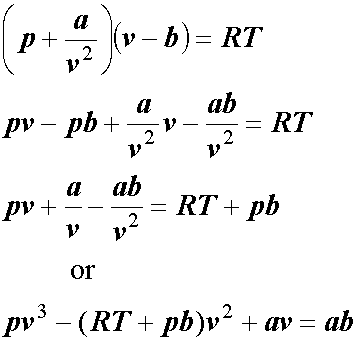Calculate the radius of He atoms if its van der Waal's constant 'b' is 24mL `"mol"^(-1)`. (Note: mL= - YouTube
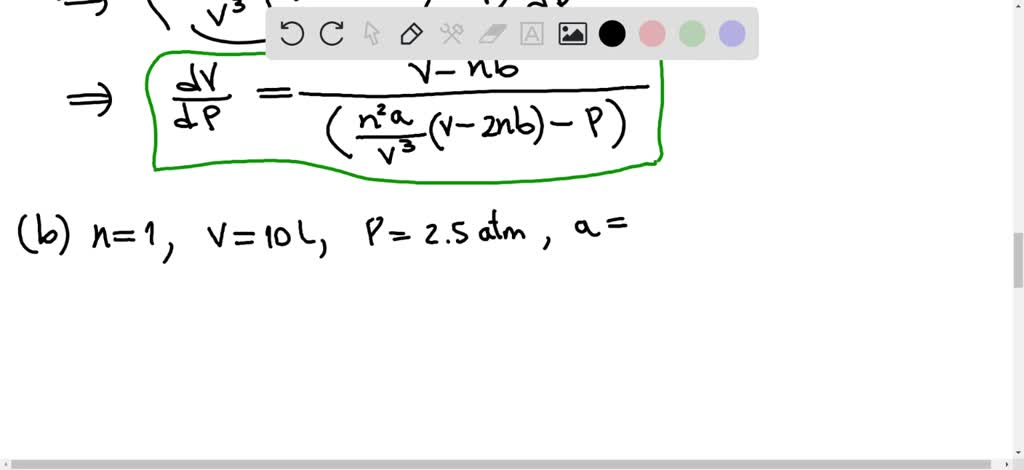
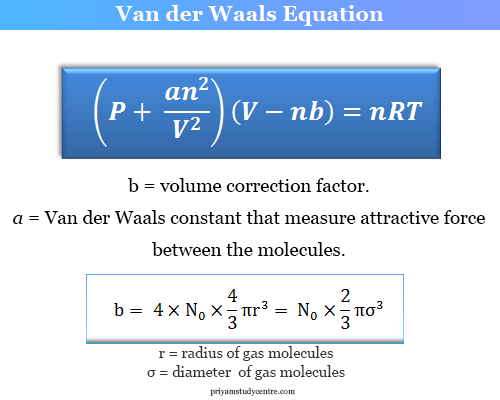
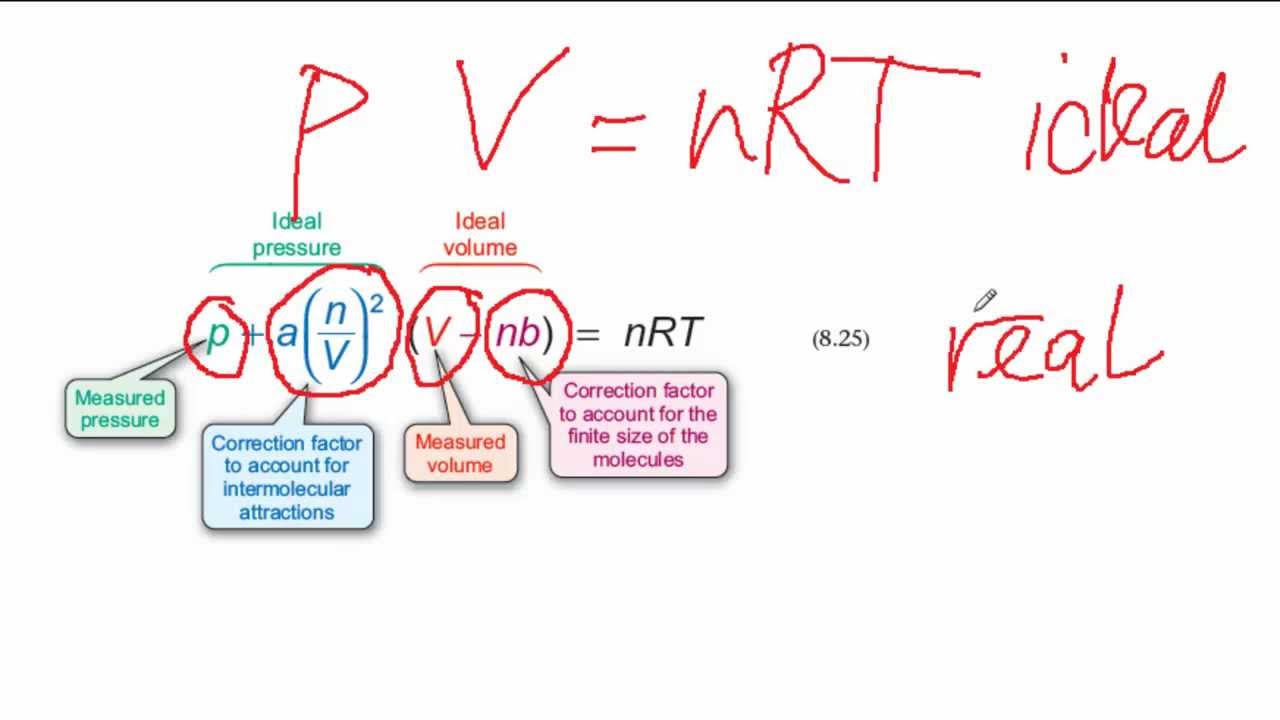
SOLVED: (a) The van van der Waals equation for n moles of a gas is (P + (n^2 a)/(V^2))(V - nb) = nRT where P is the pressure, V is the volume,
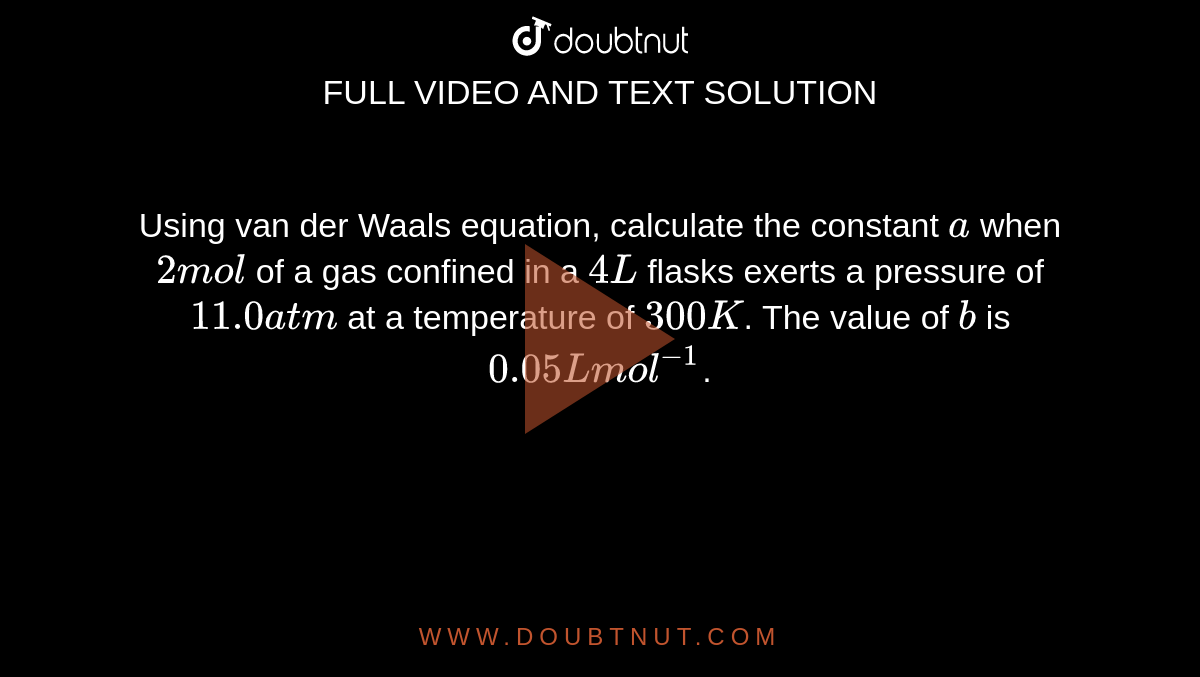
Using van der Waals equation, calculate the constant a when 2 mol of a gas confined in a 4 L flasks exerts a pressure of 11.0 atm at a temperature of 300
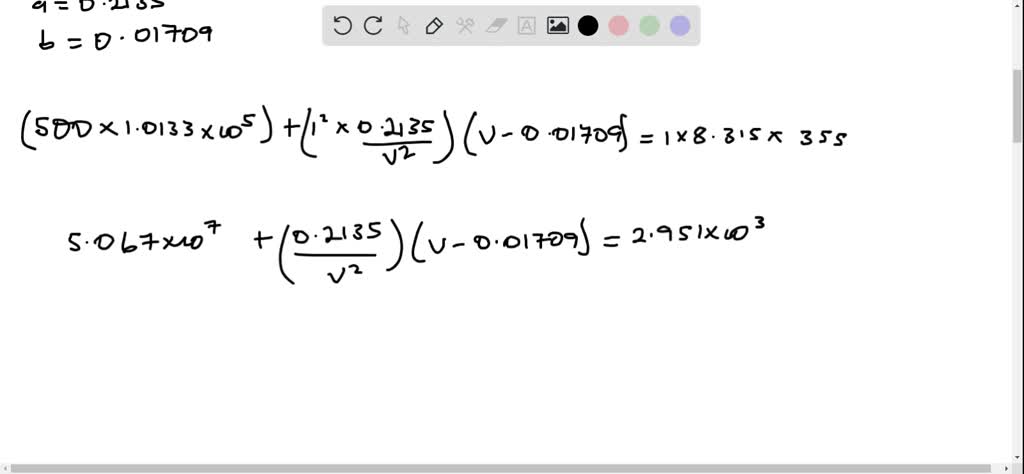
SOLVED: Use the van der Waals equation and the ideal gas equation to calculate the volume of 1.000 mol of neon at a pressure of 500.0 atm and a temperature of 355.0