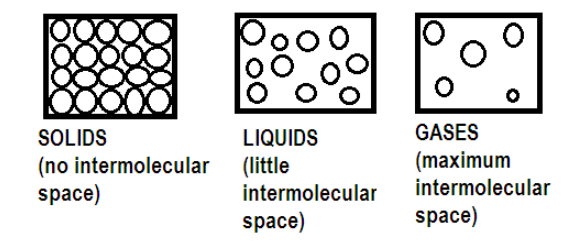
The rate of diffusion of liquids is ______.A) Higher than gases.B) Lower than solids.C) Higher than solids. D) Equal to gases.

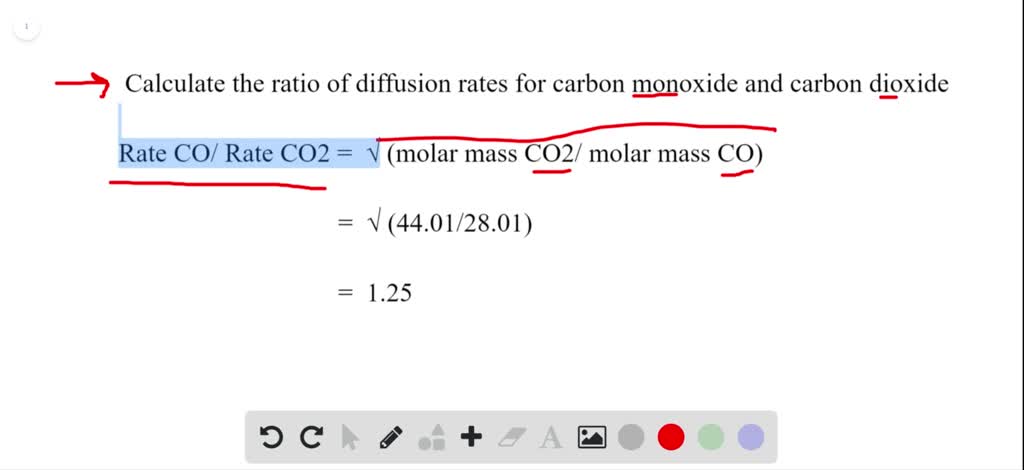
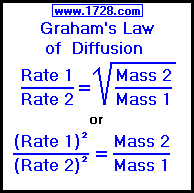
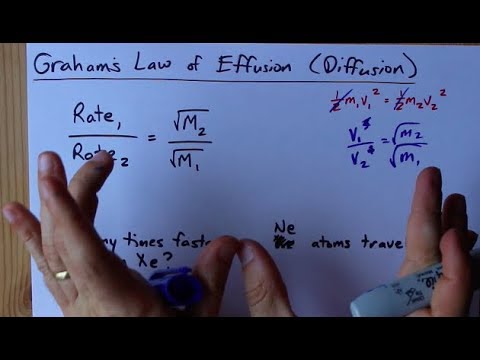
SOLVED:The rate of diffusion of a gas is proportional to: (a) (P)/(√(d)) (b) (P)/(d) (c) √((P)/(d)) (d) (√(P))/(d)
Can the rate of effusion or diffusion be negative, in accordance with Graham's law? If so, how? - Quora

Graph of diffusion constant versus temperature. Solid lines represent... | Download Scientific Diagram

Rate of diffusion of 1 mole CO and 2 mole N(2) in a container are same Rate of diffusion prop sqrt(1//M) at constant P,T .

Increasing rate of diffusion in organ systems. | Biology units, Biochemistry, Anatomy and physiology
![Development of the diffusion rate of [BMIM]Cl at different coagulation... | Download Scientific Diagram Development of the diffusion rate of [BMIM]Cl at different coagulation... | Download Scientific Diagram](https://www.researchgate.net/profile/Yumei-Zhang-8/publication/251387805/figure/fig3/AS:669046437584897@1536524509156/Development-of-the-diffusion-rate-of-BMIMCl-at-different-coagulation-temperature-with.png)
Development of the diffusion rate of [BMIM]Cl at different coagulation... | Download Scientific Diagram