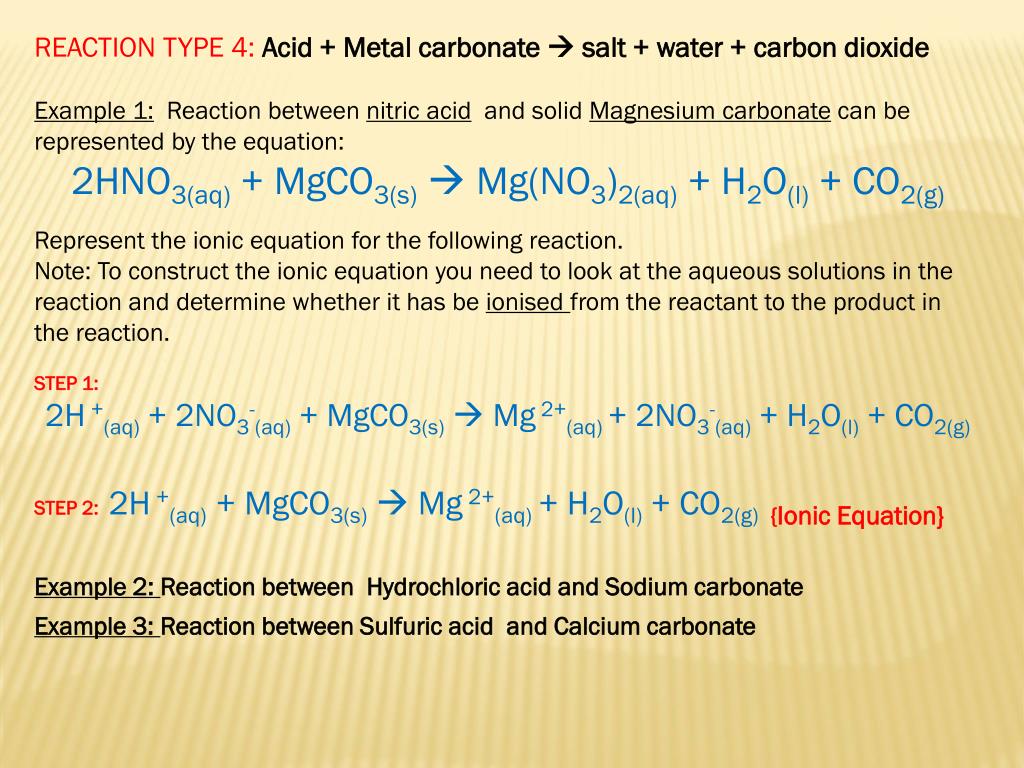
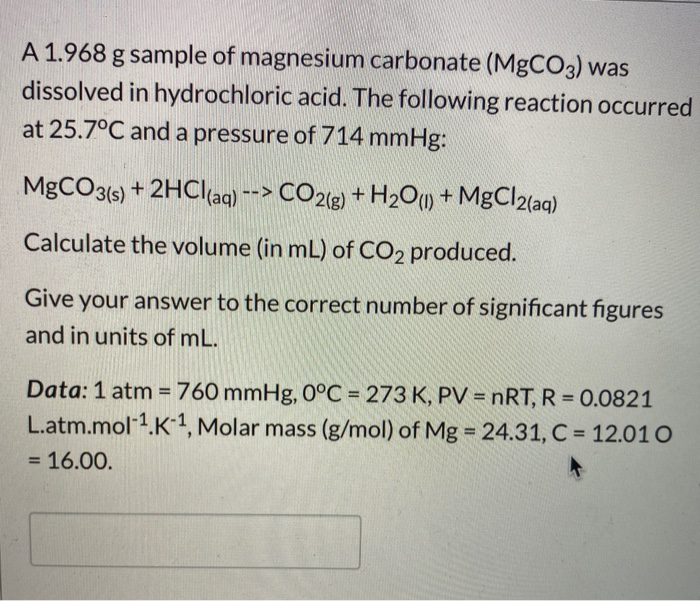
Magnesium carbonate reacts with hydrochloric acid to form magnesium chloride, carbon dioxide and water Translate and balance the equation - Science - Chemical Reactions and Equations - 12554199 | Meritnation.com

balanced equation for the following a)megnesium carbonate reacts with HCL acid to produce megnesium - Brainly.in

SOLVED:The mineral dolomite contains magnesium carbonate. This reacts with hydrochloric acid. MgCO3(s)+2 HCl(aq) →CO2(g)+MgCl2(aq)+H2 O(ℓ) (a) Write the net ionic equation for this reaction and identify the spectator ions. (b) What type
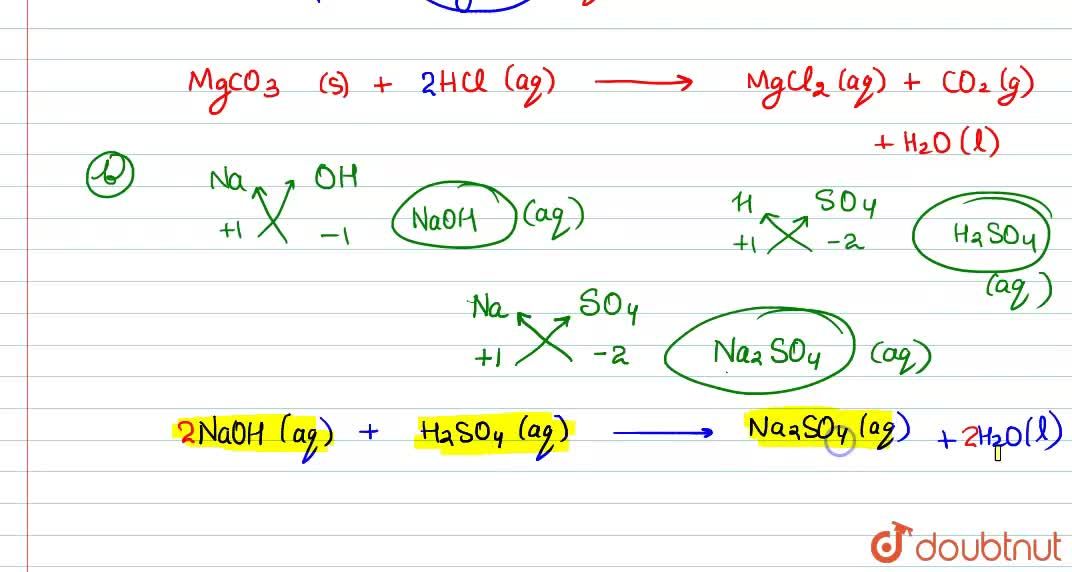
Write the balanced equations for the following reactions, and add the state symbols : (a) Magnesium carbonate reacts with hydrocloric acid to produce magnesium chloride, carbon dioxide and water. (b) Sodium hydroxide
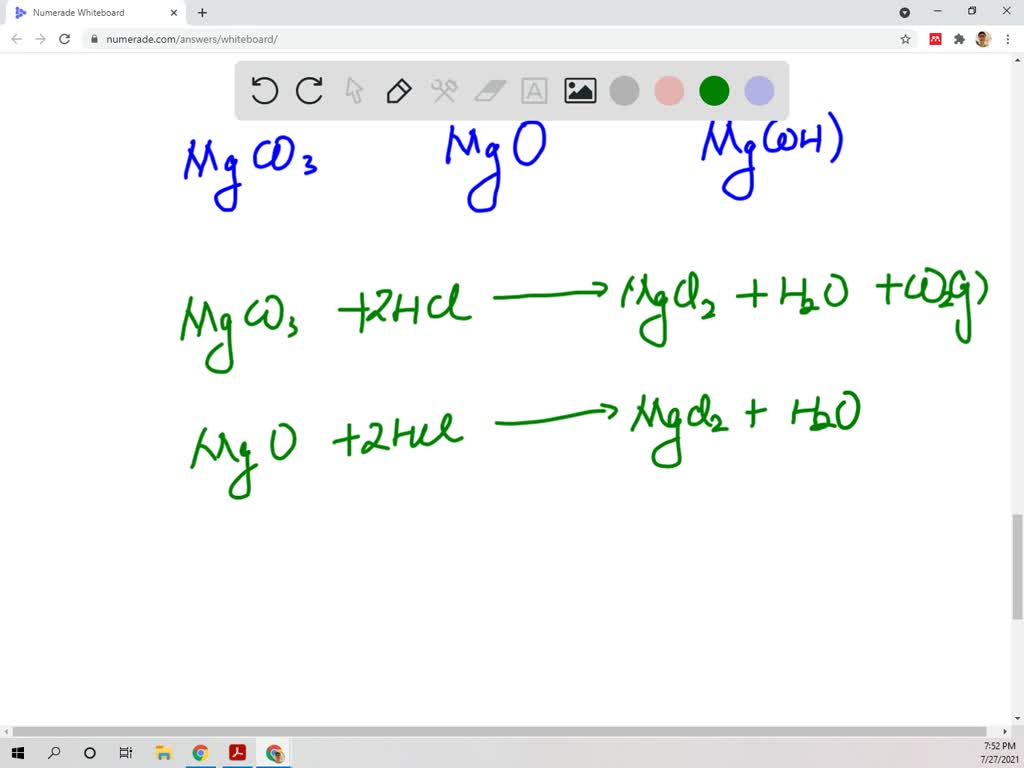
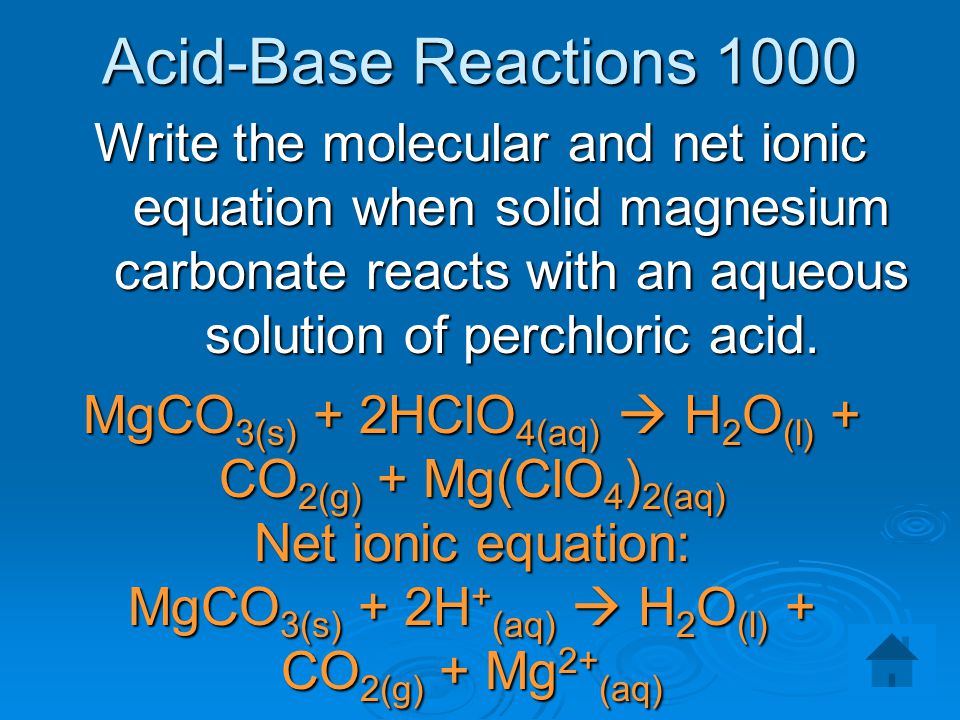
SOLVED: Magnesium carbonate, magnesium oxide, and magnesium hydroxide are all white solids that react with acidic solutions. (a) Write a balanced molecular equation and a netionic equation for the reaction that occurs

Write the balanced equations for the following reactions, and add the state symbols : (a) Magnesium carbonate reacts with hydrocloric acid to produce magnesium chloride, carbon dioxide and water. (b) Sodium hydroxide