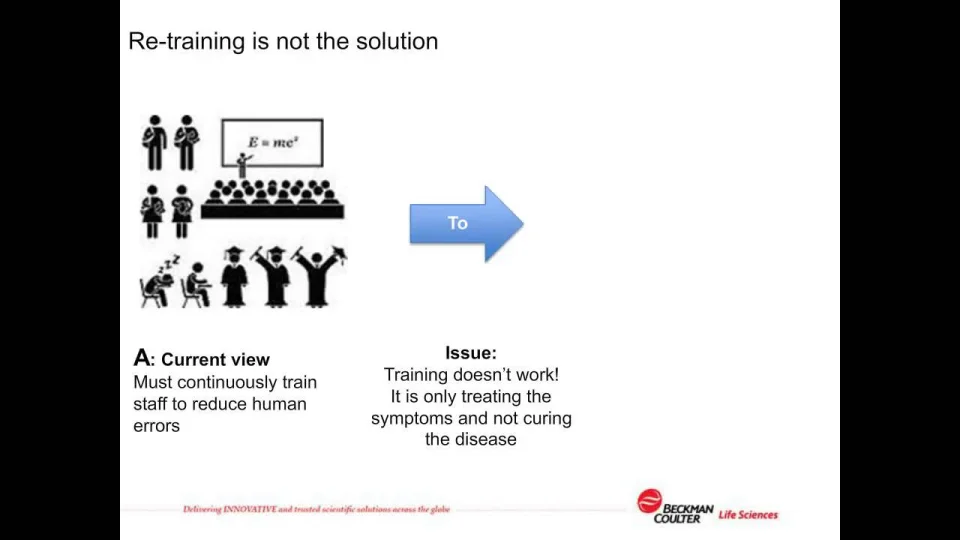
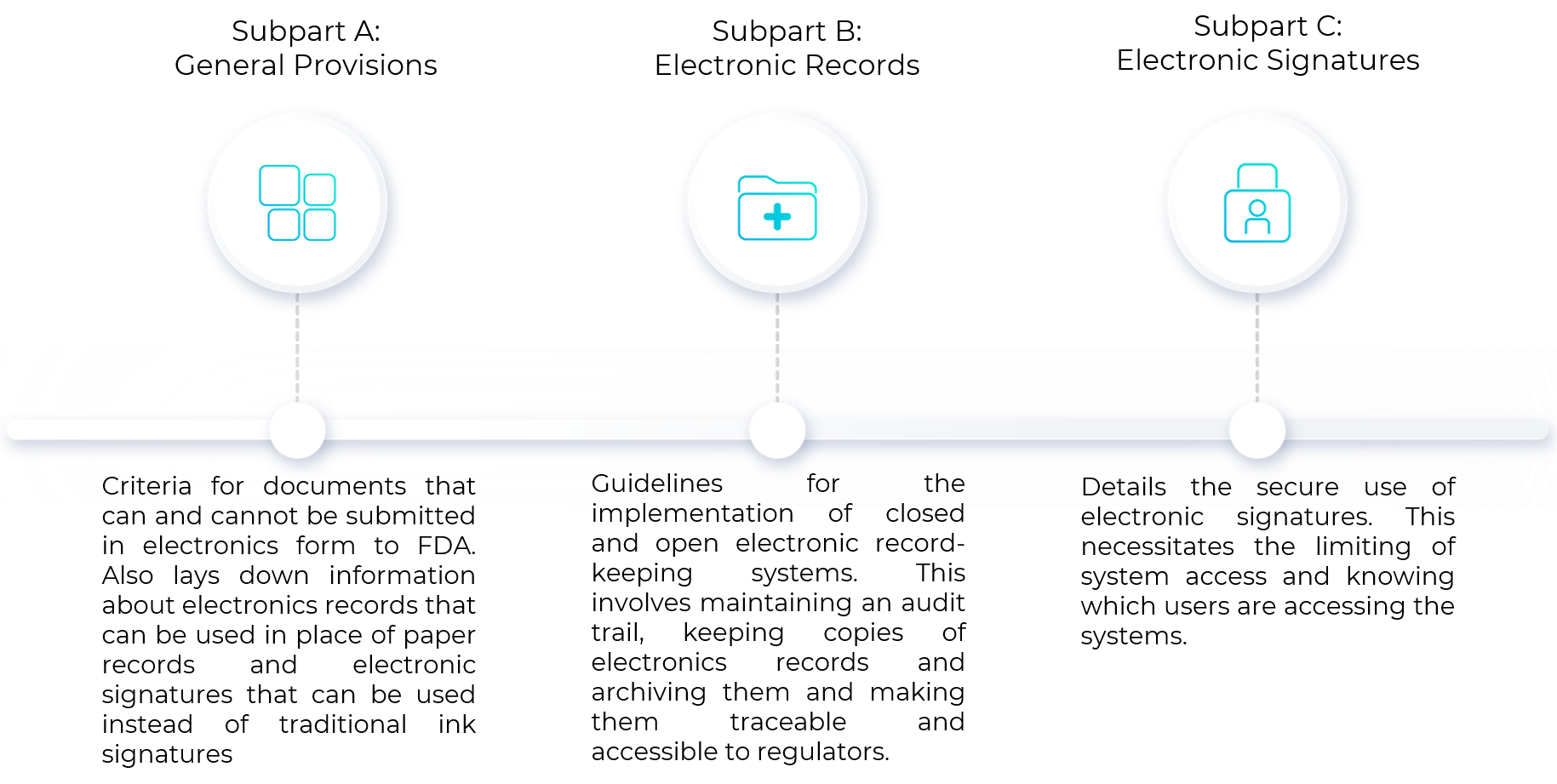
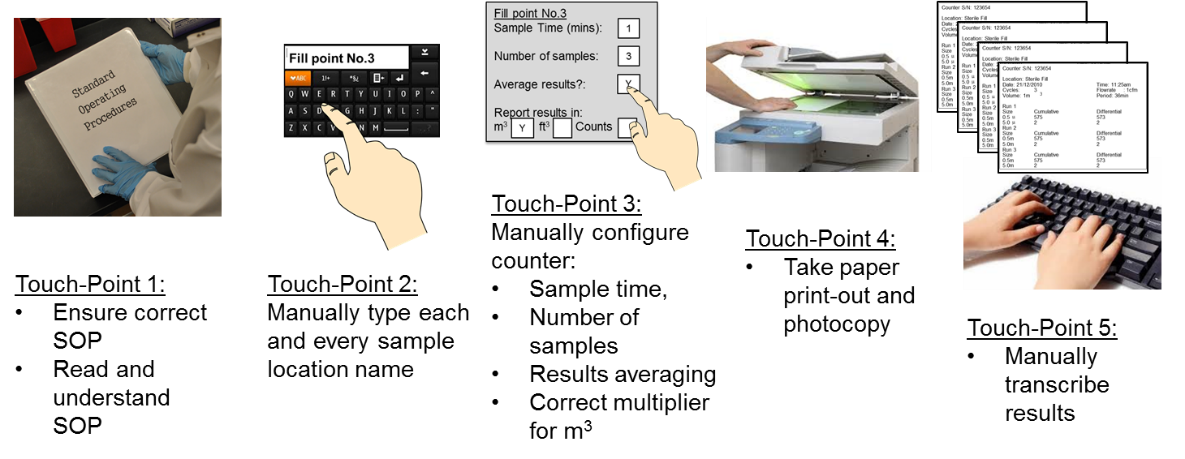
Reduce costs for compliance with data integrity: 21 CFR Part 11, SaaS/Cloud Tickets, Thu, Nov 17, 2022 at 1:00 PM | Eventbrite
![How to Avoid FDA Data Integrity System Access Warning Letters (21 CFR Part 11) [Video] - LearnGxP: Accredited Online Life Science Training Courses How to Avoid FDA Data Integrity System Access Warning Letters (21 CFR Part 11) [Video] - LearnGxP: Accredited Online Life Science Training Courses](https://learngxp.com/wp-content/uploads/2020/05/ELM-F001-06-How-to-Avoid-FDA-Data-Integrity-System-Access-Warning-Letters-21-CFR-Part-11.png)
How to Avoid FDA Data Integrity System Access Warning Letters (21 CFR Part 11) [Video] - LearnGxP: Accredited Online Life Science Training Courses

Data Integrity and Privacy – compliance with 21 CFR Part 11, SaaS/Cloud Tickets, Wed, Jan 25, 2023 at 1:00 PM | Eventbrite

DATA INTEGRITY AND PRIVACY – COMPLIANCE WITH 21 CFR PART 11, SAAS/CLOUD, EU GDPR | Data integrity, 21 cfr part 11, Webinar







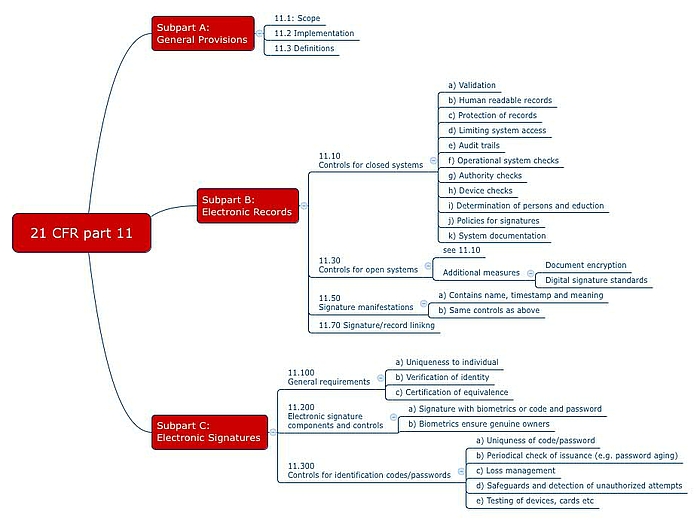




![Compliance with 21 CFR Part 11, SaaS-Cloud, EU GDPR [Data Integrity] - Austin Monthly Magazine Compliance with 21 CFR Part 11, SaaS-Cloud, EU GDPR [Data Integrity] - Austin Monthly Magazine](https://d2u8towkwolubl.cloudfront.net/wp-content/uploads/2019/07/c4all1.jpg)